Grifols demonstrates a significant reduction (61%) in the progression of moderate Alzheimer’s disease using its AMBAR treatment protocol
The combination of Plasmapheresis (a well-known and safe procedure used in plasma exchange) with Albutein® 20% (Albumin - a safe, well tolerated plasma protein with multiple properties) has demonstrated a significant reduction in the progression of the disease in the moderate AD patients participating in the study and may offer a new treatment pathway for the illness.
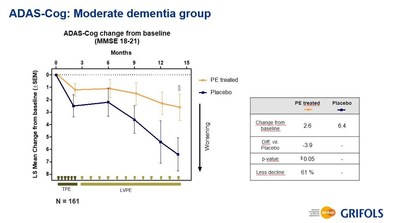
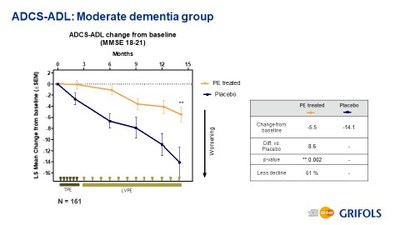
Results in the pre-specified cohort of moderate AD patients demonstrated a statistically significant reduction of 61% in disease progression from baseline across both primary efficacy endpoints as measured by the Alzheimer's Disease Assessment Scale-cognitive (ADAS-Cog) and the Alzheimer's Disease Cooperative Study – Activities of Daily Living (ADCS-ADL) scales. While a consistent delay in the progression of disease was observed in the treatment arms for the pre-specified mild cohort (the placebo arm presented a similar pattern), and the difference did not reach statistical significance.
The primary outcomes of the study were the changes from baseline to 14 months in the validated scales of cognition and activities of daily living, ADAS-Cog and ADCS-ADL, respectively. The pre-specified primary analysis was performed on the total study population and included the assessment of the differences to placebo in the primary outcomes of the following study arms: a) three combinations of plasma exchange with albumin and IGIV replacement that shared the same volume of plasma removed (plasmapheresis) regardless of the arm, b) an arm with all patients treated with plasma exchange, and c) an arm that included all patients treated with plasma exchange analyzed by disease severity: mild AD and moderate AD.
In the three-combination arms the differences to placebo showed between 50 and 75% less decline for the ADAS-Cog scale in the treated patients and between 42 and 70% less decline for the ADCS-ADL scale. In the arm with all patients treated with plasma exchange the difference to placebo achieved a 66% less decline for the ADAS-Cog scale in the treated patients with a statistical significance of 0.06 and a 52% less decline for the ADCS-ADL scale with a statistically significant value of 0.03.
In the arm with all patients treated with plasma exchange analyzed by disease severity, patients with mild AD did not decline but the same lack of progression was observed in the placebo group for both ADAS-Cog and ADCS-ADL scales, suggesting that more follow-up time is needed to observe disease progression in milder disease. Conversely, patients with moderate AD treated with plasma exchange showed a 61% less decline compared to placebo for both, ADAS-Cog and ADCS-ADL scales with statistical significances of 0.05 (significant) and 0.002 (highly significant), respectively. In addition, when moderate AD patients were analyzed by plasma exchange combination type, all three combinations achieved statistical significance for ADCS-ADL.
Fundació ACE in
"The treatment effect observed in the group with moderate severity is remarkable and those findings open new avenues for the research of neurodegenerative disorders in the adult that have the potential to offer the Alzheimer's disease patients a new modality of treatment",
"We are very pleased with the results and we celebrate them as a breath of fresh air that brings hope to the Alzheimer's patients and their families. This is the most significant development in the treatment of patients with moderate Alzheimer's disease in over 15 years," said Mercé Boada, M.D., Ph.D., Director of Fundació ACE.
"As a clinician it is also important to note that this procedure is safe and feasible. We have conducted more than 1000 plasma exchanges in our center and close to 5000 in the total study", continued
Víctor Grífols Roura, Chairman of
ABOUT AMBAR
AMBAR is an international, multicenter, randomized blinded and placebo controlled, parallel group clinical trial that enrolled mild and moderate Alzheimer patients from 41 Treatment Centers in
AMBAR was designed to evaluate whether the progression of Alzheimer's could be stabilized through plasma exchange, a process that entails periodically extracting plasma and replacing it with a specific albumin solution (Albutein®). AMBAR is based on the hypothesis that most of the amyloid-beta protein – one of the proteins accumulated in the brains of Alzheimer's patients – is bound to albumin and circulates in plasma. Extracting this plasma may flush amyloid-beta peptide from the brain into the plasma, thus limiting the disease's impact on the patient's cognitive functions. Additionally, Albumin may represent a multi-modal approach to the management of the disease due to it's binding capacity, antioxidant, immune modulatory and anti-inflammatory properties.
The AMBAR study included 496 mild and moderate Alzheimer patients, randomized in three treatment groups and one control (placebo) group. The participants were 55-85 years old and the efficacy of treatment was measured by changes in cognition and in daily living activities scores. An independent contract research organization (CRO), oversaw the trial's clinical monitoring phase and managed the data collection and analysis stages. The trial employed a randomized and double-blind design, meaning that neither patients nor evaluators knew whether subjects were receiving the treatment or the placebo.
The company began its research on Alzheimer's disease in 2004 with several pre-clinical trials, two pilot studies and a Phase II clinical trial before launching the AMBAR trial.
About Alzheimer's disease
Alzheimer's disease is a neurodegenerative pathology characterized by the death of neurons in the brain. Described as a 21st-century epidemic, it currently has no cure and its prevalence is expected to rise, especially among elderly populations in developed countries.
The most common cause of dementia, Alzheimer's causes memory loss, intellectual impairment, behavioral change, and the inability to perform daily tasks. It also has a major psychological, physical, social and economic impact on caretakers, society, and healthcare systems.
The
NOTE TO EDITORS:
Information, graphic resources and audiovisual material on the AMBAR study and Alzheimer's disease are also available on
About
With a network of 250 plasma donation centers,
In 2017, sales exceeded
The company's class A shares are listed on the
For more information, visit grifols.com.
LEGAL DISCLAIMER
The facts and figures contained in this report that do not refer to historical data are "future projections and assumptions". Words and expressions such as "believe", "hope", "anticipate", "predict", "expect", "intend", "should", "will seek to achieve", "it is estimated", "future" and similar expressions, in so far as they relate to the
References
1. http://www.who.int/news-room/fact-sheets/detail/dementia
View original content to download multimedia:http://www.prnewswire.com/news-releases/grifols-demonstrates-a-significant-reduction-61-in-the-progression-of-moderate-alzheimers-disease-using-its-ambar-treatment-protocol-300738956.html
SOURCE






FEMA approves individual assistance to seven more Georgia counties
Tiny district, high stakes: Mattiello-Frias race a referendum on the status quo
Advisor News
- Flexibility is the future of employee financial wellness benefits
- Bill aims to boost access to work retirement plans for millions of Americans
- A new era of advisor support for caregiving
- Millennial Dilemma: Home ownership or retirement security?
- How OBBBA is a once-in-a-career window
More Advisor NewsAnnuity News
- An Application for the Trademark “DYNAMIC RETIREMENT MANAGER” Has Been Filed by Great-West Life & Annuity Insurance Company: Great-West Life & Annuity Insurance Company
- Product understanding will drive the future of insurance
- Prudential launches FlexGuard 2.0 RILA
- Lincoln Financial Introduces First Capital Group ETF Strategy for Fixed Indexed Annuities
- Iowa defends Athene pension risk transfer deal in Lockheed Martin lawsuit
More Annuity NewsHealth/Employee Benefits News
Life Insurance News
- Private placement securities continue to be attractive to insurers
- Inszone Insurance Services Expands Benefits Department in Michigan with Acquisition of Voyage Benefits, LLC
- Affordability pressures are reshaping pricing, products and strategy for 2026
- How the life insurance industry can reach the social media generations
- Judge rules against loosening receivership over Greg Lindberg finances
More Life Insurance News